Clinical Trials
Participating in Clinical Trials
Every day, research uncovers new information about epilepsy and seizures. Your involvement in clinical trials could help in the development of new medications or medical devices. You and many others may benefit from your willingness to become involved.

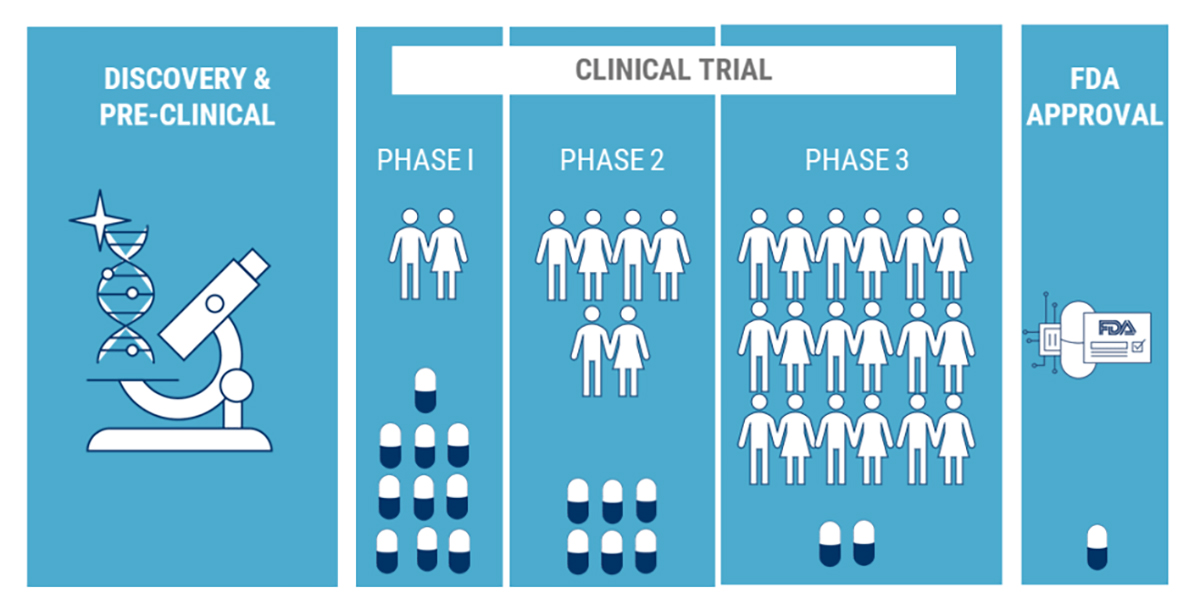
Volunteering to participate in a clinical trial is one of the best ways you can contribute to the understanding of epilepsy. Volunteers are vital to the clinical trial phase of the drug approval process. As a volunteer, you are the most critical link in a long chain of research and testing in the development of new medications.
The benefits of volunteering to participate in a clinic trial include:
- Participating in a research process that may lead to new therapies.
- Obtaining expert medical care with physicians that specialize in epilepsy and seizures during the trial.
- Learning new information about your condition.
- Gaining access to new research treatments before they become available to the public.
How Does Minnesota Epilepsy Group Protect Patients?
When conducting clinical trials, Minnesota Epilepsy Group’s most important responsibility is to protect patients through well-designed protocols, oversight and approval by a a dedicated Institutional Review Board (IRB) and a thorough informed consent process.
Clinical Trial Protocol
A protocol is a detailed plan that explains what will be done in a clinical trial and why. The plan is carefully designed to protect your health and welfare while addressing specific research questions. It outlines how many patients will participate, what medical tests they will receive and how often, and the treatment and monitoring plan. Research staff follows the protocol approved by the Institutional Review Board (IRB).
Who Is the Institutional Review Board?
An Institutional Review Board (IRB) is a group of healthcare professionals and members of the local community that review and approve a clinical trial before it begins. The IRB carefully reviews clinical trial activity. The primary responsibility of the IRB is to protect the safety and rights of clinical trial participants.
What Is Informed Consent?
Informed consent is the process designed to give volunteers the information they need to decide about participating in a clinical trial. This process allows the volunteer to ask questions and to exchange information freely with the clinical investigator. The clinical investigator is responsible for ensuring the informed consent is obtained from each research volunteer before that person participates in the clinical trial. The consent is not a contract. You may decide not to participate at any time during the trial.
If you agree to participate in a clinical trial after learning all that is involved, then you, the trial’s principal investigator (or nurse, if called for in the protocol) and a witness will sign and date the informed consent form. A copy of the form will be given to you.
Currently Enrolling Research Studies at Minnesota Epilepsy Group
Please note that we are actively recruiting for the following studies even if Minnesota Epilepsy Group is not listed as such on the study’s ClinicalTrials.gov web page:
Pediatric Studies
A Phase 2B, Multicenter, 30-week, Prospective, Cross-over, Double-blind, Randomized, Placebo-controlled Study Followed by a 52-Week Open-Label Extension Study to Evaluate the Efficacy and Safety of Basimglurant Adjunctive to Ongoing Anticonvulsive Therapy in Children and Adolescents with Uncontrolled Seizures Associated with Tuberous Sclerosis Complex
https://clinicaltrials.gov/ct2/show/NCT05059327?term=NOEMA&draw=2&rank=1#contacts
• 5-18 years of age with diagnosis of Tuberous Sclerosis Complex
• Currently on at least 1 AED and failed at least 2 AEDs
• Must have had between 3 and 20 seizures per month over last year and at least 5 seizures within past 30 days.
Adult Studies
A Phase 2/3 Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy, Safety and Tolerability of BHV-7000 in Subjects with Refractory Focal Onset Epilepsy
https://clinicaltrials.gov/study/NCT06132893?term=BHV-7000%20refractory%20focal&rank=2
• 18-75 years of age with focal onset seizures
• Currently on 1-3 AEDs
• Must be having 4 or more focal motor seizures per month
Adult and Pediatric Studies
Human Epilepsy Project 3: Treatment Responsive Idiopathic Generalized Epilepsy (Cohort 2)
• 13+ years of age / Age of seizure onset between 8 and 30 years
• 2+ years of well-controlled seizures
• No convulsive seizures for 2+ years
• Ongoing therapy with 1+ Anti-seizure Medication
Human Epilepsy Project 3: Treatment Resistant Idiopathic Generalized Epilepsy (Cohort 3)
• 13+ years of age / Age of seizure onset between 8 and 30 years
• 2+ years on anti-seizure medication(s)
• Trialed and failed 2+ anti-seizure medications
• Either one or both of the following:
o -1+ GTC per year for the past 2 years
o -Any type of seizure at least every 3 months
• Seizures were not caused by significant illness or missed medications
A Phase 3b/4, Interventional, Multicenter, Open-Label, Single-Arm Study to Assess Behavioral and Other Co-Occurring Outcomes Following Treatment With EPID(I/Y)OLEX as Add-on Therapy in Participants (Aged 1 to 65 Years Old) With Seizures Associated with Tuberous Sclerosis Complex (EpiCom)
https://clinicaltrials.gov/study/NCT05864846?term=JZP926-402&rank=1
• 1-65 with confirmed TSC diagnosis
• Behaviors that are considered moderate to severe
• Must be taking 1 or more AEDs and have had a current or past history of seizures
If you are interested in learning more about participating in an epilepsy research study at Minnesota Epilepsy Group, please email Sarah Ellis at sellis@mnepilepsy.net or call our office at (651) 241-5290 and ask for Sarah Ellis.
