Deep Brain Stimulator (DBS)
On April 27, 2018 FDA approved DBS for adult (18y and older) patients with refractory focal onset (Partial) epilepsy. The use of DBS in epilepsy may be recent but its use in neurology is not. It has been successfully used for several types of movement disorders now for several years.
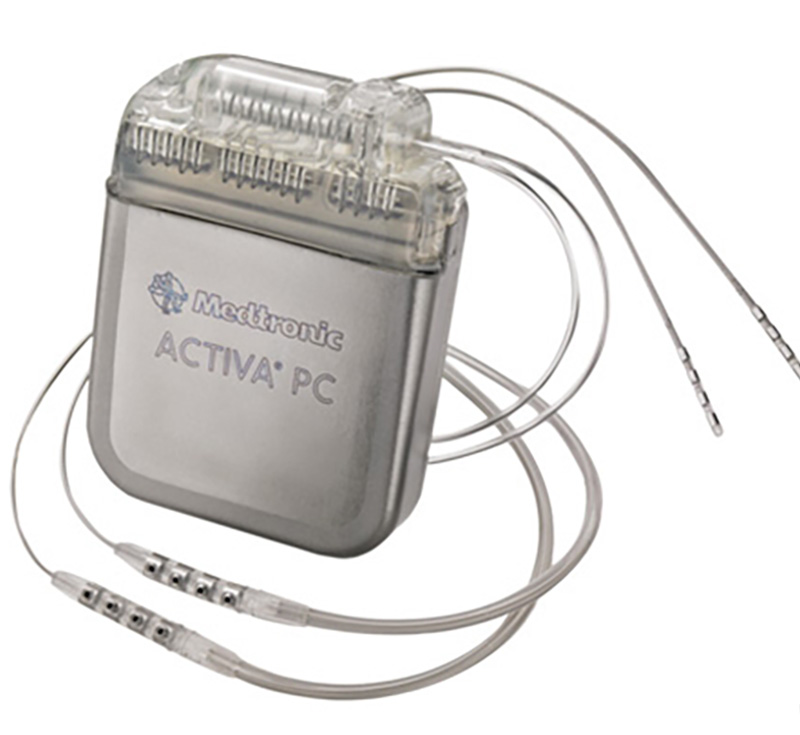
How it Works
DBS works on principles similar, but not identical, to VNS. DBS aims to regularly electrically stimulate small parts of a deep part of the brain called thalamus. Thalamus (Thalamii plural), one on each side of the brain, act as the control center of the brain. Much of the information going into the brain finds its way to the thalamus. Among its several other functions the thalamii also help connect and regulate different parts of the brain. For epilepsy, DBS targets the front end of the thalamus called anterior nucleus of thalamus (ANT) on each side.
Similar to the VNS, a small device with an inbuilt battery (“generator”) is placed under the skin of the chest. Using very think wires placed under the skin, the generator is then connected to small probes placed deep in the brain. The deep end of the probes reach the ANT on each side. Though it requires surgery of the brain, this surgery involves only drilling very small holes in the skull and then advancing the probes (or ‘electrodes’) from the holes to the thalamus. Patients typically do not need an incision/cutting of the skull (craniotomy). By stimulating the ANT, the brain activity gradually changes in such a way that seizures can be reduced. Like the Vagus Nerve Stimulator, the Deep Brain Stimulator is programmed to stimulate at a pre-programmed set time, rate, and intensity.
In the clinical trials, DBS demonstrated average reduction of seizures by nearly 40% by 3 months from device placement. On longer follow-up nearly 70% average reduction in seizures was reported at 5 years. Sixty-eight percent of patients reported seizure reduction by more than half at 5 years. The variations in seizure occurrence also tend to stabilize over time after DBS placement
As mentioned above, DBS has been used for several years in patients with difficult to control movement disorders. It has proven to be safe in those patients. Surgery related complications including infection, bleeding in to the brain may occur in small number of patients. Nearly 1/4th of patients may notice stimulation related sensations.
Salanova V. Deep brain stimulation for epilepsy. Epilepsy Behav. 2018 Nov;88S:21-24.
Li MCH, Cook MJ. Deep brain stimulation for drug-resistant epilepsy. Epilepsia. 2018 Feb;59(2):273-290.
